The Effect of Jeopgol-Tang 2.0 on Recovery of Bone Fracture in Sprague-Dawley Rats
Ⓒ The Society of Pathology in Korean Medicine, The Physiological Society of Korean Medicine
Abstract
Natural Korean medicine has already been a great success, being used to treat various types of othopedic diseases, especially fracture and osteoporosis. In this study, we find a new mixture of herbal medicine (JGT 2.0) of five well-known Korean herbal medicine for bone healing, including Angelica gigas, Dendrobium moniliforme, Dipsaci Radix, Antler, Astragalus membranaceus, Conidium officinale, Achyranthes japonica, Messa medicate fermentata on bone fracture healing. To evaluate the efficacy of JGT 2.0, the ulna of Sprague-Dawley rats was intentionally fractured and administered JGT 2.0, followed by radiographic imaging and histological examination using H&E staining to confirm the progress of recovery over time at the fracture site. X-ray imaging showed no significant difference between groups up to 1 week after JGT 2.0 administration. However, after 4 weeks of JGT 2.0 administration, bone regeneration decreased by -15% in the control group, but increased by 23.6% in the JGT 2.0 group. In addition, the length of calluses increased by 252% in the JGT 2.0 group compared to the control group. Taken together, the results suggest that JGT may offer a novel therapeutic approach to accelerate the growth of bone calluses and promote healing of bone fractures.
Keywords:
Bone fracture, JGT 2.0, X-ray, RatIntroduction
A bone fracture is a partial or completely break bone. Most of bone fracture are caused by car accidents or sport injuries, but fractures may also occur due to diseases such as osteoporosis or tumors, or accumulated fatigue due to continuous vigorous exercise1,2). Fractures heal by three stages: an inflammatory phase, a restoration phase, and a remodeling phase3). When a fracture occurs, not only bone tissue but also surrounding soft tissues are damaged, and a hematoma was formed in the fractured area due to rupture of surrounding blood vessels4).
Bone tissue newly formed after a fracture is formed by the differentiation of osteogenic cells derived from the surface of the epiosteum or endosteum or by the differentiation of fibroblasts in the connective tissue around the bone into osteogenic cells by appropriate stimulation5,6). During the fracture healing process, various factors related to bone formation affect the healing rate, but the related changes in indicators of bone metabolism are not well known. In the bone formation process or calcification mechanism, there are intramembranous ossification and endochondral ossification, and inorganic salts are deposited together with the action of osteoblasts and osteoclasts to change into hard bones7-9). When a fracture occurs, active proliferation of osteogenic cells present in the periosteum at the shortest distance from each fracture surface occurs, the periosteum becomes thick, and at this time, capillaries appear around it, and the proliferation of osteogenic cells becomes more vigorous1,10,11) differentiates into osteoblasts and secretes bone matrix on the cut bone surface to form osteogenic tissue12,13).
Our previous study reported that the JGT improved low mineral density in a middle-aged man14). They took two packs of master (120mL/pack) every morning and evening. After taking JGT, calluses and bone cross-linking were confirmed in the follow-up image, and patients described their experience of pain reduction in post-recovery interviews. However, in this study, we found a new mixture of herbal medicine (JGT 2.0) of five well-known herbal medicine for bone healing, including Angelica gigas, Dendrobium moniliforme, Dipsaci Radix, Antler, Astragalus membranaceus, Conidium officinale, Achyranthes japonica, Messa medicate fermentata. To investigate the effects of JGT 2.0, the ulna of Sprague-Dawley rats was intentionally fractured, followed by administration of JGT or JGT 2.0, followed by radiographic and histological examination to confirm the progress of recovery over time at the fracture site.
Materials and Methods
1. Prescription of Jeopgol-tang (JGT) and JGT 2.0 (Table 1.)
Dried herb samples (Human Herb, Daegu, Korea) were immersed in 10 volumes of dH2O and boiled at 80℃ or for 2hours, and then the water extract was collected. The above process was repeated once, and the extracts were combined, concentrated with a rotary evaporator, and then vacuum-dried to yield 27.78 % (w/w) of the extract.
2. Animals
All experiments on animals were conducted with the approval of the Ethics Committee of Daegu Haany University (DHU2021-020) and in accordance with the US National Institutes of Health "Guide for the Care and Use of Laboratory Animals" (NIH Publication no. 80-23, revised 1996).
42 Sprague-Dawley male rats (Hyochang Sci., Daegu, Korea) that weighed 230-250 g (6 weeks old) each were used for the experiments. Male mice were group housed (three per cage) with an inverted light/dark cycle (light on from 07 : 00-19 : 00 hr). The room temperature was 22 ± 2℃ and the humidity was 50 ± 15 %. The rats had free access to food and water. All rats were handled daily for at least one week prior to the experiment. Rats were randomly divided in two groups: 1) Control: ulna bone fracture operation and no treatment and 2) JGT: ulna bone fracture operation + treatment of JGT (10 mg/kg) and 3) JGT 2.0: ulna bone fracture operation + treatment of JGT 2.0 (10 mg/kg). Experimental schedule was presented in Fig. 1.
3. Bone defect model
The rats were anesthetized with Ketamine HCl (Keaset 10 %; Fort Dodge, IO50501 USA, 85 mg/kg, i.p.) and Xylazine (XYL-M 2 %; VMD n.v., Berendonk 74-B-2370, Arendonk, Blegium, 3 mg/kg, i.p.). After cleanly shaving the right forelimb, a skin incision of about 3 cm was made and the bone was cut about 2-3 mm using osteotomy. After cutting the bone, the skin was sutured again.
4. Bone damage range
Bone damage range shown on x-ray film was measured once a week using dial calipers (dial calipers, OZAKI MGF.Co., Ltd, Japan). Bone gap immediately after surgery is set to “0”.
5. Measurement of callus formation
After oral administration of JGT or JGT 2.0 for 4 weeks, 2 mm of the anterior and posterior parts were extracted centering on the fracture line of the ulna and H&E staining was performed. All calluses were fixed in 4 % buffered formaldehyde for 48 h and then decalcified in 7 % nitric acid solution for 1 week. After that, demineralized tissues were washed for 5 min, dehydrated in alcohol and then, embedded in paraffin wax. All sample cut into 4 μm thick sections. These sections were stained with hematoxylin and eosin. The stained area was observed with an Olympus microscope (BX53, Miami, USA) to measure the length of the regenerated callus from the fracture line. As the callus is formed and bone is regenerated, the value increases.
6. Statistical analysis
The data were analyzed using the one-way ANOVA test followed by the LSD post-hoc test using the SPSS 15.0 program (P < 0.05). All the data were marked mean ± S.E., and the significance level between groups.
Results
1. The bone length growth
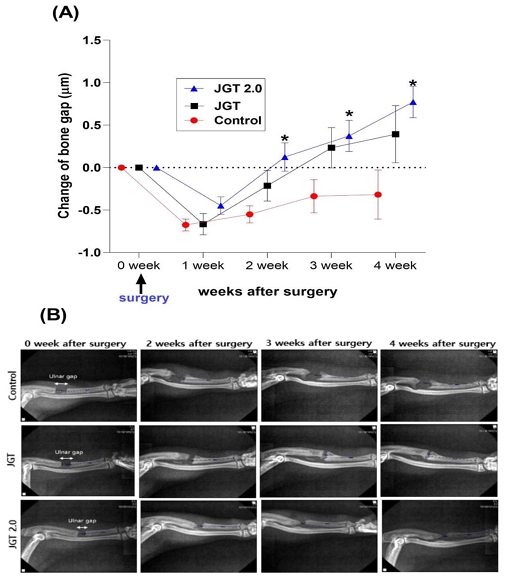
As shown in Fig. 2(A) and (B), x-ray imaging was performed weekly to measure the ulnar gap. In observing the x-ray images of bene setting progress for 4 weeks, bone length growth is rated -0.67 ± 0.07 ㎛ in control group, -0.67 ± 0.12 ㎛ in JGT group and -0.44 ± 0.10 ㎛ in JGT group starting from the first week. Until 1 week after administration during the experiment, there was no significant difference among groups. After 2 weeks, bone length growth significantly progressed in JGT 2.0 group compared to the control group (F (6, 75) = 1.843, P < 0.05). After administration of JGT or JGT 2.0 for 4 weeks, bone length growth showed -0.32 ± 0.29 ㎛ in control group 0.39 ± 0.33 ㎛ in JGT group and 0.77 ± 0.18 ㎛ in JGT 2.0 group. The recovery rate observed through x-ray images shows that the JGT 2.0 group recovers three times fater than the control group (P < 0.05).
2. The length of the callus at the fracture line
The histological result was shown in Fig. 3(A) and (B). In the histological photographs, at week 4 post-fracture, the control group exhibited the domination of callus hyperplasia along with the appearance of the woven bone, whereas a larger woven bone was developed in the JGT 2.0 group. Also, the length of the callus in JGT 2.0 group was increased by 252 % compared to that of control (P < 0.001). This result proved that JGT 2.0 has improved greatly bone healing and bone regeneration.
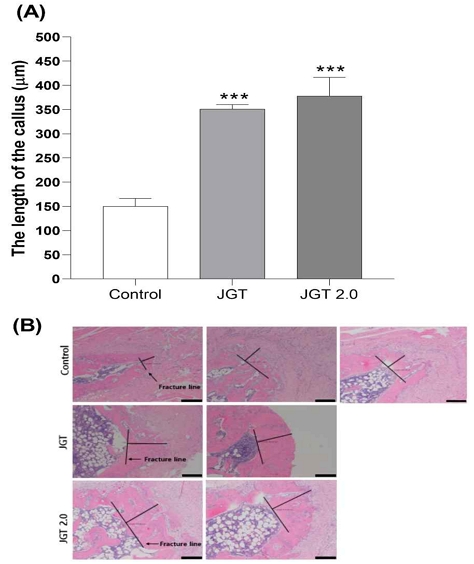
The representative histological photograph of peri-fracture tissues (A) and the length of the callus at the fracture line (B) Histological evaluation of fracture. H&E staining of fracture callus sections. Magnification, ×40; scale bar = 200 µm. Data represent the mean ± SEM. ***P < 0.001 compared with Control.
Discussion
After 2 weeks, bone recovery was significantly progressed in the JGT 2.0 group compared to the control group. After 4 weeks of JGT 2.0, bone length growth increased significantly after 3 times compared to the control group. On histological photography, 4 weeks after fracture, the control group showed dominance of callus hyperplasia with the appearance of the weaving bone, whereas in the JGT 2.0 group, a larger weaving bone developed. These results suggest that administration of JGT 2.0 may help speed up bone healing and bone regeneration.
A fracture is a break in a bone, similar to a crack or break. Bones can be completely fractured or partially fractured in several ways. Also, fractures take weeks to months to heal, depending on the seveerity of the injury and how well you follow treatmet. Healing fractures requires surgery, slings, braces, or other devices, as well as proper nutrition and overall health. However, natural oriental medicine has alrealy achieved great success in treating many types of orthopedic conditions, including osteoporosis, bone fracture, and rheumatism15). In additiohn to fundamental treamtnet of freactures, a healing process that can reduce recovery time is necessary for patients to quickly recover from their daily lives16).
Our previous study proved that the JGT treatment improved the bone mineral density (BMD) in 44-year-old male who had BMD of in lumbar spine scan14) and also JGT prescription was useful for treatment of delayed bone union17).However JGT 2.0 was excluded Albizia Julibrissin Durazz because it is hepatotoxic18). Also, Deer antler was added based on research results showing that deer antler is more effective in treating osteoporosis than deer antler19). this study, five well-known herbal medicine for bone healing, including Angelica gigas, Dendrobium moniliforme, Dipsaci Radix, Antler, Astragalus membranaceus, Conidium officinale, Achyranthes japonica, Messa medicate fermentata. Angelica gigas, were newly discovered a mixture (JGP 2.0). Antler has the effects of nourishing the blood, relieving pain by activating blood circulation, and increasing intestinal energy absorption, and is used for symptoms, such as syndrome of heart-liver blood deficiency, menopause, afterpain, contusion injury, pain caused by a large abscess, and constipation20,21). Dendrobium moniliforme has the effects if promoting the secretion of gastric juice by strengthening the stomach, and removing fever by cultivating yin energy, and is used to treat symptoms such as thirst caused by fever22,23). Dipsaci Radix has an effect of strengthening the liver and kidney and connecting broken bones or muscles and is used to treat symptoms such as the lack of proper blood of the liver and kidney and back and leg pain24,25). Antler is used in the present invention is obtained by cutting and drying the ossified horns of Cervus Nippon Temmnick, Cervus elaphus Linne or Cervus Canadensis Erxleben belonging to the family Cervidae. The antlerhas the effects of nourishing the vitality of the kidneys, boosting yang energy, promoting bone growth by promoting the synthesis of protein and nucleic acids, promoting hematopoiesis and is known to improve immune function and to be effective against liver disease and osteoporosis26) Astragalus membranaceus is known to have a remarkable effect pm the treatment of knee diseases (arthritis, rheumatoid arthritis, and inflammation causes by bruises). It is also frequently used in the case in which the waist and leg feel heavy27,28). Cnidium officinale is used in boosting yang qi, protecting and firming the skin, removing toxins such as inflammation, giving new flesh, facilitating moisture drainage, and removing edema, and is used to treat symptoms such as spleen Gi deficiency and symptoms of weakness in both nasal and lungs19,29). Achyranthes japonica is used to relieve cerebral blood vessel spasms while lowing blood pressure when there are symptoms such as headache, dizziness, and eye irritation in hypertension30-32). Messa medicate fermentata mainly acts on the spleen and stomach to strengthen the function of the digestive system, has an action to comfort the stomach with the action to help digesting, and exhibits effects against an upset stomach, a stuff chest, vomiting and diarrhea and abdominal pain due to extravasated blood after childbirth33). Antler is used for treatment of osteoporosis34). Also, polysaccharide of Astagalus promote angiogenesis via activation of TLR4 signaling pathway35). Another study also proved the isoflavone of Astragalus membranaceus has prevented osteoporosis development36). Park et al reported that the Achyranthes japonica can help bone density in asovariectomized induced osteoporotic mouse model37). Achyranthes japonica inhibit osteoblast and osteoclast cells in ovariectomized mice38). Consistant with previous study, after 4 weeks of JGT 2.0 administration, bone length growth significantly progressed in JGT 2.0 group compared to the control group. After 4 weeks of JGT 2.0 administration, bone regeneration showed a decrease of -15% in the control group, but the bone regeneration rate increased by 23.6% in the JGT 2.0 group. Also, histochemical result showed that at week 4 post-fracture, the control group exhibited the domination of callus hyperplasia along with the appearance of the woven bone, whereas a larger woven bone was developed in the JGT 2.0 group. The length of the callus in JGT 2.0 group was increased by 252 % compared to that of control. The recovery process of a fracture proceeds in the following order: inflammatory phase, repair phase, and remodeling phase. During the inflammatory phase, the bone cells that have fallen from the fracture site are absorbed, so the fracture surface becomes wider when seen on an x-ray. Next, during the recovery period, the hematoma changes into soft callus and the fracture interval decreases. JPT 2.0 group recovered the inflammatory phase quickly, and produced soft callus faster compared to the JPT group. Taken together, these results assume that JGT 2.0 may be effective in reducing bone damage during the early inflammatory phase of fracture and promotes bone healing after restoratinocaused by fractures via activation of cartilage callus formation.
To our knowledge, this will be the first pre-clinical trial to evaluate the efficacy of JGT 2.0 in an animal model of bone fracture. The results of this study are expected to contribute to the development of new herbal medicine mixture that can be used for bone regeneration treatment.
The limitation of this study is that the small number of samples, and bone density measurement and morphological observation were not performed for elucidation of the effect of JGT 2.0 on bone fracture recovery mechanism. However, that X-ray examination showed that the bone regeneration rate of JGT 2.0 treated group was markedly faster than that of the control group that is considered to have a positive effect on fracture treatment.
References
-
Camal, Ruggieri IN, Ccero AM, Issa JPM, Feldman S. Bone fracture healing: perspectives according to molecular basis. J Bone Miner Metab. 2021;39:311-31.
[https://doi.org/10.1007/s00774-020-01168-0]

-
Ural A. Advanced Modeling Methods-Applications to Bone Fracture Mechanics. Curr osteoporos Rep. 2020;18(5):568-76.
[https://doi.org/10.1007/s11914-020-00615-1]

-
Ma CH, Chiu YC, Tu YK, Yen CY, Wu CH. Three-stage treatment protocol for recalcitrant distal femoral nonunion. Arch Orthop Trauma Surg. 2017;137:489-98.
[https://doi.org/10.1007/s00402-017-2634-x]

-
Schell H, Duda GN, Peters A, Tsitsilonis S, Johnson KA, Schmidt BK. The haematoma and its role in bone healing. J Exp Orthop. 2017;4
[https://doi.org/10.1186/s40634-017-0079-3]

-
Mathieu V, Fukui K, Matsukawa M. Micro-Brillouin scattering measurements in mature and newly formed bone tissue surrounding an implant. J Biomech Eng. 2011;133.
[https://doi.org/10.1115/1.4003131]

-
Roschger A, Wagermaier W, Gamsjaeger S. Newly formed and remodeled human bone exhibits differences in the mineralization process. Acta Biomater. 2020:104;221-30.
[https://doi.org/10.1016/j.actbio.2020.01.004]

-
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nature reviews. Rheumatology. 2012;8:133-43.
[https://doi.org/10.1038/nrrheum.2012.1]

-
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nature reviews. Rheumatology. 2015;11:45-54.
[https://doi.org/10.1038/nrrheum.2014.164]

-
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551-5.
[https://doi.org/10.1016/j.injury.2011.03.031]

-
Bahney CS, Zondervan RL, Allison P. Cellular biology of fracture healing. J Orthop Res : official publication of the Orthopaedic Research Society. 2019;37:35-50.
[https://doi.org/10.1002/jor.24170]

-
Zhang Y, Xu J, Ruan YC. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22:1160-9.
[https://doi.org/10.1038/nm.4162]

-
Cottrell JA, Turner JC, Arinzeh TL, O'Connor JP. The Biology of Bone and Ligament Healing. Foot Ankle Clin. 2016;21:739-61.
[https://doi.org/10.1016/j.fcl.2016.07.017]

-
Schlundt C, El KT, Serra A. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2018;106:78-89.
[https://doi.org/10.1016/j.bone.2015.10.019]

-
Kim J, Lee BC, Lee H. Improvement of Low Bone Mineral Density Treated with Jeopgol-tang in a Middle-Aged Man: A Case Report. J Korean Med. 2021;42;90-7.
[https://doi.org/10.13048/jkm.21018]

-
Peng Z, Xu R, You Q. Role of Traditional Chinese Medicine in Bone Regeneration and Osteoporosis. Front Bioeng Biotechnol. 2022;10
[https://doi.org/10.3389/fbioe.2022.911326]

-
Singh V. Medicinal plants and bone healing. Nat J Maxillofac Surg. 2017;8:4-11.
[https://doi.org/10.4103/0975-5950.208972]

-
Won J, Choi Y, Yoon LS, Lee JH, Choi K et al. Individualized herbal prescriptions for delayed union: A case series. Explore (New York, N.Y.) 2023;19:260-6.
[https://doi.org/10.1016/j.explore.2022.03.001]

-
Jing J, Teschke R, Traditional Chinese medicine and herb-induced liver injury: comparison with drug-induced liver injury. J Chin Transl Hepatol. 2018;6:57-68.
[https://doi.org/10.14218/JCTH.2017.00033]

- Meng HY, Qu M, Li XB, Yuan N, Lin Z. Effective of piolse antler and antler glue on osteoporosis of ovariectomized rats. Zhong yao Cai. 2009;32:179-82.
-
Ji KY, Jung DH, Pyun BJ, Klim YJ, Lee JY, Choi S, et al. Angelica gigas extract ameliorates allergic rhinitis in an ovalbumin-induced mouse model by inhibiting Th2 cell activation. Phytomedicine. 2021;93
[https://doi.org/10.1016/j.phymed.2021.153789]

-
Kwon DA, Kim YS, Kim SK, Baek SH, Kim HK, Lee HS. Antioxidant and antifatigue effect of a standardized fraction (HemoHIM) from Angelica gigas, Cnidium officinale, and Paeonia lactiflora. Pharmaceut Biol. 2021;59:391-400.
[https://doi.org/10.1080/13880209.2021.1900878]

-
Baek JM, Kim JY, Ahn SJ. Dendrobium moniliforme Exerts Inhibitory Effects on Both Receptor Activator of Nuclear Factor Kappa-B Ligand-Mediated Osteoclast Differentiation in Vitro and Lipopolysaccharide-Induced Bone Erosion in Vivo. Molecules (Basel, Switzerland). 2016;21:295
[https://doi.org/10.3390/molecules21030295]

-
Lee YJ, Kim JH, Kim Y. Dendrobium moniliforme Stem Extract Inhibits Lipoteichoic Acid-Induced Inflammatory Responses by Upregulation of Heme Oxygenase-1. J Microbiol Biotechnol. 2018;28:1310-7.
[https://doi.org/10.4014/jmb.1807.07024]

-
Wong RW, RabieAB, Hägg EU. The effect of crude extract from Radix Dipsaci on bone in mice. Phytother Res : PTR. 2007;21:596-8.
[https://doi.org/10.1002/ptr.2126]

-
Wu H, Lv Y, Wei F, Li C, Ge W, Du W. Comparative analysis of anti-osteoporosis efficacy in Radix Dipsaci before and after processing with salt based on spectrum-effect relationship. J Pharm Biomedical Anal. 2022;221
[https://doi.org/10.1016/j.jpba.2022.115078]

-
Xia P, Liu D, Jiao Y. Health Effects of Peptides Extracted from Deer Antler. Nutrients. 2022;14.
[https://doi.org/10.3390/nu14194183]

-
Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. AM J Chin Med. 2016;44:1-22.
[https://doi.org/10.1142/S0192415X16500014]

-
Fu J, Wang Z, Huang L. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res. 2014;28:1275-83.
[https://doi.org/10.1002/ptr.5188]

-
Lee YM, Lee YR, Kim CS. Cnidium officinale extract and butylidenephthalide inhibits retinal neovascularization in vitro and in vivo. BMC complement Alt Med. 2016;16:231.
[https://doi.org/10.1186/s12906-016-1216-8]

- Lee GH, Hwang KA, Kang JH, Choi KC. Effect of Achyranthes japonica Nakai extract on immunity and anti-inflammation in dogs. Can J Vet Res = Revue canadienne de recherche veterinaire. 2020;84:294-301.
-
Liu X, Lee SI, Kim IH. Achyranthes japonica extracts supplementation to growing pigs positively influences growth performance, nutrient digestibility, fecal microbial shedding, and fecal gas emission. Animal bioscience. 2021;34:427-433.
[https://doi.org/10.5713/ajas.20.0012]

-
Muniyappan M, Jeon SY, Choi MK, Kim IH. Dietary inclusion of Achyranthes japonica extract to corn-soybean meal-wheat-based diet on the growth performance, nutrient digestibility, cecal microflora, excreta noxious gas emission, and meat quality of broiler chickens. Poultry science. 2022;101.
[https://doi.org/10.1016/j.psj.2022.101852]

-
Fu FQ, Xu M, Wei Z, Li W. Biostudy on Traditional Chinese Medicine Massa Medicata Fermentata. ACS omega. 2020;5:10987-94.
[https://doi.org/10.1021/acsomega.0c00816]

-
He J, Li X, Wang Z. Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front Pharmacol. 2019;10:1344.
[https://doi.org/10.3389/fphar.2019.01344]

-
Qiu H, Zhang L, He X, et al.: Promotion of angiogenesis in vitro by Astragalus polysaccharide via activation of TLR4 signaling pathway. J food biochem, 46, e14329, 2022.
[https://doi.org/10.1111/jfbc.14329]

-
Kaczmarczyk-Sedlak I, Wojnar W, Zych M, Ozimina-Kamińska E, Taranowicz J, Siwek A. Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evidence-based complementary and alternative medicine : eCAM. 2013;457052.
[https://doi.org/10.1155/2013/457052]

-
Park E, Lee CG, Kim J. Anti-Osteoporotic Effects of the Herbal Mixture of Cornus officinalis and Achyranthes japonica In Vitro and In Vivo. Plants (Basel, Switzerland). 2020;9.
[https://doi.org/10.3390/plants9091114]

-
Park E, Kim J, Yeo S. Anti-Osteoporotic Effects of Combined Extract of Lycii Radicis Cortex and Achyranthes japonica in Osteoblast and Osteoclast Cells and Ovariectomized Mice. Nutrients. 2019;11.
[https://doi.org/10.3390/nu11112716]