Comparative Study of White, Red, and Black Ginseng Extract on Improves the Learning and Memory Impairments by Increases of Synaptic Protein Expression in Scopolamine-induced Dementia Rats
Ⓒ The Society of Pathology in Korean Medicine, The Physiological Society of Korean Medicine
Abstract
To compare and analyze the improvement effects of white ginseng extract, red ginseng extract, and black ginseng extract on cognitive dysfunction and memory impairment caused by scopolamine in rats. In the cognitive behavioral test, the tendency of the SCOP+B group to overcome the escape time delay induced by scopolamine administration was observed, unlike the SCOP group. The frequency on plat form was significantly increased in the group treated with ginseng extracts compared to the SCOP group. As a result of measuring the duration time on goal quadrant, the time spent in the quadrant was significantly increased in the SCOP+B group compared to the SCOP group. In the hippocampus, the SCOP-treated group significantly decreased the activity of AChE compared to the normal group, but the ginseng extract-treated groups significantly increased it compared to the SCOP group. After sacrificing the rats after the behavioral test, the expression of PSD95 protein in the excised brain was significantly decreased in the SCOP group compared to normal, but it was observed that the SCOP+R and SCOP+B groups were significantly increased compared to the SCOP group. CREB1 protein expression was significantly increased in the SCOP+R group, and the expression of Cdk5 was significantly increased in the SCOP+B group. Ginseng extracts significantly restored the memory damaged by scopolamine suggesting that red ginseng increased the expression of CREB1 and PSD95 proteins, and black ginseng increased the protein expression of Cdk5 and PSD95 to induce memory recovery.
Keywords:
Postsynaptic density protein 95 (PSD95), Cyclic adenosine monophosphate responsive element-binding 1 (CREB1), Cyclin-dependent kinase 5 (Cdk5), Scopolamine, Ginseng extractIntroduction
Since the 21st century, our society has faced various medical problems as life expectancy increases and the aging society enters. In particular, the prevalence of dementia and Alzheimer's disease continued to increase, reaching 959,001 in 2019 and medical expenses also increased to 16.5 trillion won. In general, cognitive function gradually increases from childhood to adulthood, peaks at some point in adulthood, and then begins to decline toward old age1). Therefore, in our society ahead of an aging society and ahead of an aging society, interest in cognitive function and memory improvement is continuously increasing, and the importance of related research is also being emphasized.
On the other hand, the increase of the elderly population has increased interest and demand for health functional food, independent of the quality improvement and nutritional enhancement of food, and health functional food is becoming a very important part for modern people2). As a result, the health functional food industry in Korea has grown at an average annual rate of about 7.4% since 2011. Among them, ginseng-related products account for about 40% of the market share.
Ginseng has been traditionally used for health promotion and treatment for over 2000 years in many Asian countries. It is mainly used in the form of white ginseng and red ginseng. White ginseng is used after drying ginseng, and red ginseng is steamed and dried3). Ginseng contains ginsenosides, ployacetylenes, and phenolic compounds. Among them, ginsenoside is known as an important active substance for cognitive activity4).
Unprocessed ginseng is called fresh ginseng because its water content reaches 75%. Since fresh ginseng is difficult to store fresh for more than several days, and there is a risk of deterioration during distribution, methods for processing fresh ginseng have been developed for a long time. White ginseng is a form of fresh ginseng dried with hot air, fresh ginseng is dried by boiling with water, and red ginseng is steamed and dried with steam. Until now, studies on the composition and pharmacological effects of ginseng have been mainly limited to fresh ginseng, white ginseng, and red ginseng, and very few studies have been done on black ginseng5).
Mechanisms for improving cognitive function and memory of ginseng include increased cardiovascular and cerebral blood flow, neuroprotective effects, secondary effects due to hypoglycemic effects, and neurotransmitter control. The fact that red ginseng has a positive effect on cognitive function improvement is also known through various animal and clinical trials6). However, research on specific brain regions of ginseng components for cognitive enhancement is still lacking. In particular, the effect of black ginseng, a new type of ginseng, on cognitive function improvement has not been confirmed yet.
In this study, based on the study on the memory improvement effect of red ginseng, we tried to confirm the memory improvement effect of black ginseng, and to investigate the mechanism of action in the brain by measuring the expression of proteins related to neuroprotection and synapse reinforcement.
Materials and Methods
1. Animal
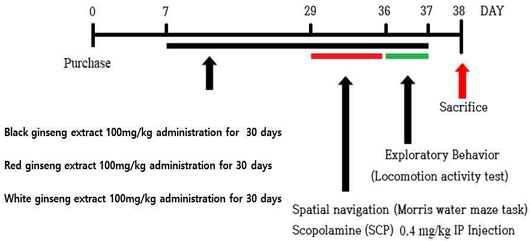
As experimental animals, 7-week-old male Sprague-Dawley (SD) rats were purchased (SamTaco Co. Osan, Korea) and used. The rats were acclimatized for one week in a controlled laboratory breeding environment (12 h light/dark cycle, room temperature 22±2℃) for one week, and then used for the experiment. After measuring the body weight, the weight was divided into 4 groups by the Latin square method. 8-week-old rats were treated as a control group (Normal), a group administered with scopolamine alone (SCOP, 0.4 mg/kg, IP injection), and a group administered with scopolamine and ginseng extracts such as SCOP+R, SCOP+B and SCOP+W (100 mg/kg, daily oral administration for 30 days) shared Scopolamine was administered for 8 days during the water maze test. This study complied with the Bioethics Act and ethical guidelines for the use of laboratory animals and was conducted under the approval of the Laboratory Animal Ethics Committee of Wonkwang University (WKU16-49).
2. Ginsengs extraction
Ginsengs are made with 4-year-old ginseng purchased from the local Jinan Red Ginseng Research Institute (Jinan, Korea). White ginseng was prepared by drying at 60℃, and Black ginseng was prepared by steaming white ginseng at 98℃ for 3 hours and drying it at 60℃ for 18 hours repeatedly 3 times. Red ginseng was prepared by steaming white ginseng at 98℃ for 3 hours and drying it at 60℃ for 18 hours. To prepare the ginseng extract, ginseng was ground and sonicated in 10 volumes of 80% ethanol at 50℃ for 1 hour 3 times, then filtered and freeze-dried.
3. Morris water maze test
Through the underwater maze experiment, the cognitive function enhancement and spatial perception ability of experimental animals were measured. Seven days out of a total of 8 days of experimentation was a training session, and after the training period was over, a probe test was conducted. The experimental equipment was carried out in a water tank with a diameter of 180 cm and a height of 60 cm. An escape platform made of acrylic was fixed in one of the quadrants, and the surface was designed to be invisible by immersion 1 cm below the water surface. During the training period, the experimental animals were asked to swim for 60 seconds at different locations from 4 different directions 4 times a day to find the escape zone. Therefore, consideration was given to learning about spatial perception. The time to reach the escape zone was recorded and measured using an Ethovision system (Noldus Co., Spain). The proof experiment for underwater maze learning was conducted once for 60 seconds after the escape zone was removed on the 8 days of the Morris water maze experiment. Scopolamine (0.4 mg/kg, I.P. injection) was administered 30 minutes before the Morris water maze experiment. The Normal group was orally administered with distilled water in the same volume as the black ginseng administration group.
4. Open field test
Exploratory behavior test was performed to measure situational learning and anxiety behavior of experimental animals. After exposing the experimental animals to an open space in a square acrylic box (45 x 45 cm), their search behavior was observed for 5 minutes. In the search behavior box, infrared sensors were installed on the x and y axes at an interval of 2.54 cm, and the behavior of the experimental animals was measured using an activity monitor (Activity Monitor ENV-520; Med Association Inc., VT, USA).
5. Acetylcholinesterase (AChE) activity in hippocampus
Collect and pellet the hippocampus tissue by centrifugation and remove the supernatant. Wash the tissues 3 times with PBS then resuspend in PBS. Lyse the tissues by ultrasonication 4 times. Alternatively freeze the tissues to -20℃ and thaw to room temperature 3 times. Centrifuge at 1500g×g for 10 min at 2-8℃ to remove cellular debris. Collect the supernatant for assaying. Store samples at -20℃ or below. Add 100 μl of sample, standard, or blank to each well and incubate for 2 hours at 37℃. Aspirate and add 100 μl of detection reagent A and incubate of 1 hour at 37℃. Aspirate and wash 3 times. Add 100 μl of detection reagent B and incubate for 10-20 min at 37℃. Aspirate and wash 5 times. Add 90 μl of TMB substrate solution and incubate for 10-20 min at 37℃. Add 50ul of stop solution, read immediately at 450nm.
6. Western blot
After the behavioral experiment, the hippocampus was dissected and separated to measure protein expression in the brain of the experimental animal. The isolated hippocampus was washed twice with cold PBS, and then put in lysis buffer (50 mM HEPES, 150 mM NaCl, 1% deoxycholate, 1 mM EDTA, 1 mM PMSF and 1 μg/ml aprotinin, pH 7.4) and dissolved. After incubating the sample on ice for 1 hour, the tissue was centrifuged at 10,000 rpm at 4℃. for 30 minutes, and the supernatant was separated and collected. The protein concentration of the supernatant was determined using the Bradford method. For Western blot analysis, the same amount of protein was electrophoresed on 10% or 15% SDS-PAGE gel, and then transferred to a PVDF membrane. Thereafter, the PVDF membrane was blocked with 5% skim milk dissolved in PBS for 90 minutes and washed with PBST. Membranes were blocked with PBS containing 5% bovine serum albumin for 1 h at room temperature and then incubated with primary antibodies including anti-β-actin and anti-PSD95, anti-CREB1, and anti-Cdk5 (1:1000 dilution, Cell Signaling, Beverly, MA, USA), overnight at 4℃, followed by horseradish peroxidase-conjugated Anti-rabbit secondary antibody (1:1000 dilution, Cell Signaling) for 1 h at room temperature. Peroxidase activity was estimated using an enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific, Waltham, MA, USA).
7. Statistical analysis
The behavioral test results were expressed as mean ± standard error, and n = 6 or more per experimental group. Neurochemical experiments using hippocampal tissue were performed three times, and the results were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare data between groups, and when a statistically significant difference occurred, a post-hoc test was analyzed using Fisher's PLSD. Significance was set at p < 0.05. GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis.
Results
1. Ginseng extracts supplementation improves scopolamine-induced memory and learning impairment
The water maze experiment is widely used to evaluate hippocampal-dependent long-term and spatial memory. As a result of measuring the mean escape latency for the experimental animals to reach the shelter, at the beginning of the training period, the SCOP group, SCOP+R, SCOP+B, and SCOP+W groups were compared to the normal group during the training period (training session), there was no difference until the 3 days (Fig. 2). However, on the 4 days, compared to the Normal group, it was observed that the time of the remaining groups receiving scopolamine was delayed. However, in the SCOP+R group, the escape time was significantly shorter than in the SCOP group on the 4 days, but from the 5 days, the time was delayed similarly to the SCOP group (Fig. 2A). In the SCOP group, the escape time was significantly delayed even after the 4 days, compared to the normal group, but unlike the SCOP group, the escape time delay induced by scopolamine administration was overcome in the SCOP+B group, significant difference was observed in the first day compared to the SCOP group (Fig. 2B).
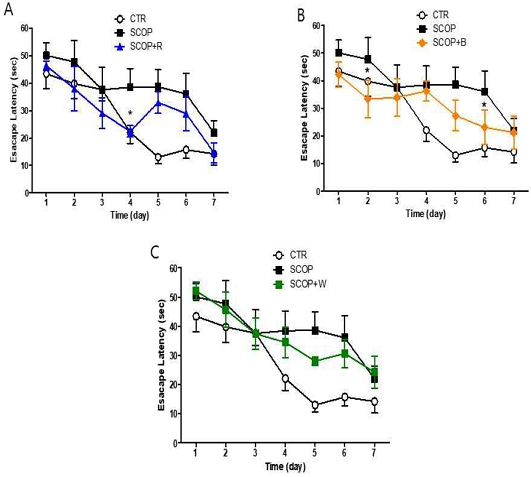
Measurement of the mean escape latency on training session in Morris water maze task. Data are expressed as the mean ± S.E.M. (n = 6), * p <0.05 vs the SCOP group by ANOVA (post-hoc test with Fisher's PLSD)
In the verification experiment after the training period, the frequency on plat form was significantly reduced in the SCOP group than in the normal group (Fig. 3). However, in SCOP+R group, SCOP+B, and SCOP+W group, it increased significantly with SCOP group (Fig. 3A-3C). Also, because of measuring the duration time on goal quadrant where the probe was located, the SCOP group showed a significant decrease compared to the normal group, but SCOP+R and SCOP+W showed an increasing pattern compared to the SCOP group. There was no significant. However, the time spent in the quadrant significantly increased in the SCOP+B group compared to the SCOP group (Fig. 3D). It was confirmed that in SCOP+B group, compared to SCOP group, it was confirmed that the sheltered location was remembered and the time to search for and stay there increased.
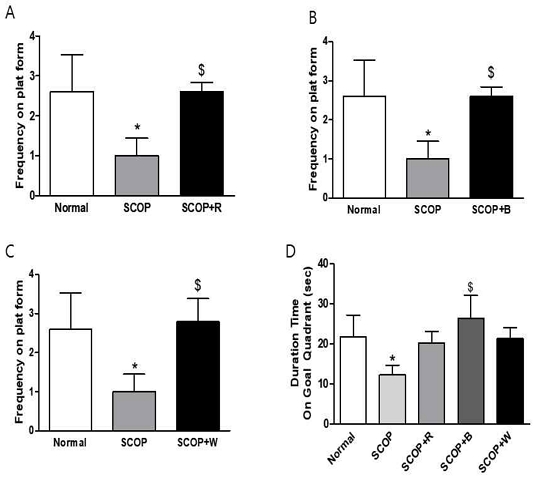
Measurement of the accurately crossing frequency on plat form exist area in the probe test after training session(A-C) and mean represents spend time in probe-test (D) in Morris water maze test. Data are expressed as the mean ± S.E.M. (n = 6), * p <0.05 vs Normal group, $ p <0.01 vs the SCOP group by ANOVA.
2. Open-field test
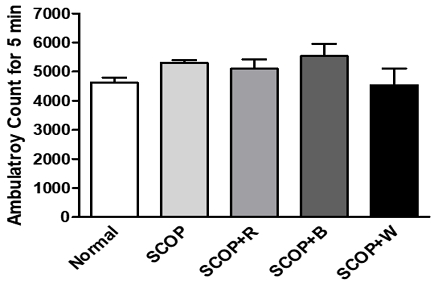
Gait activity was measured through the exploratory behavior test. An open-field test was conducted to determine whether long-term oral administration of ginsengs influences exploratory behavior and anxiety in a scopolamine-induced memory loss model. In a new environment, rats perform search behavior for situational learning, and the degree of search behavior can be confirmed by measuring the observed locomotive activity. As a result of the experiment, there was no significant difference in the number of gaits between the normal group and the ginseng-treated group for 5 minutes (Fig. 4).
3. Ginseng extracts significantly improve the Acetylcholinesterase (AChE) activity in the Hippocampus of scopolamine-induced memory and learning impairment
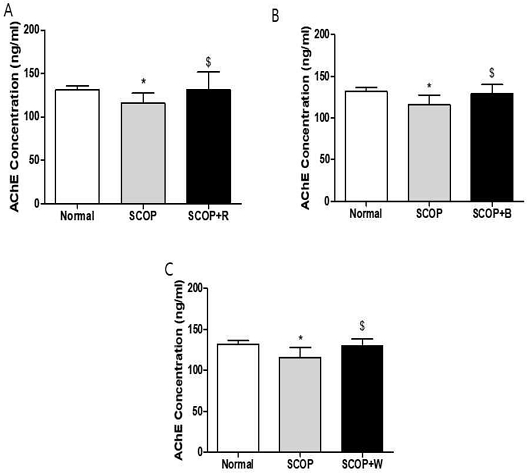
After sacrificing the rats after the behavioral test, the hippocampus was isolated from the extracted brain and AChE was measured. In the hippocampus, the SCOP-treated group significantly decreased AChE activity compared to the normal group (Fig. 5), but increased significantly in the SCOP+R, SCOP+B and SCOP+W groups compared to the SCOP group (Fig. 5).
4. Ginseng extracts significantly increases of synaptic protein expression in the Hippocampus of scopolamine-induced memory and learning impairment
After sacrificing the rats after the behavioral test, the hippocampus was isolated from the brain, and postsynaptic density protein 95 (PSD95), an intracellular structural protein that plays an important role in synapse formation and function, and cyclic adenosine monophosphate (cAMP) responsive element-binding 1 (CREB1) and Cyclin-dependent kinase 5 (Cdk5), which are known to play an important role in memory formation, were measured. In the hippocampus, PSD95 protein expression was significantly decreased in the SCOP group compared to normal (Fig. 6A-6C), but a marked increase was observed in the SCOP+R and SCOP+B groups compared to the SCOP group (Fig. 6A and 6B). In the hippocampus, CREB1 protein expression was not changed in the SCOP group compared to the normal group, but a marked increase was confirmed in the SCOP+R group (Fig. 6D). Also, as a result of confirming the expression of Cdk5 in the hippocampus, it decreased compared to normal in the SCOP group, but increased significantly in the SCOP+B group (Fig. 6E).
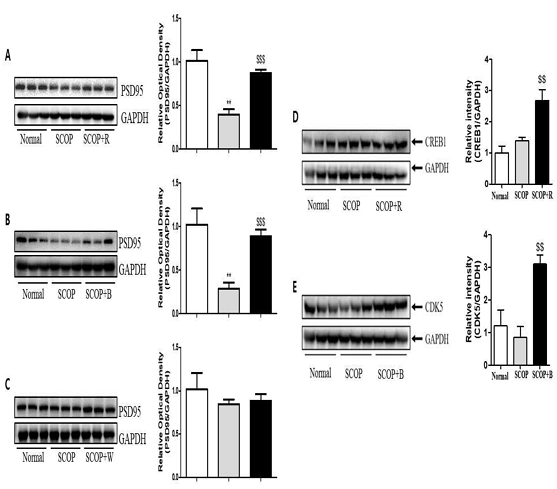
Representative pictures of Western-blot images and measurement of relative optical densitometry of PSD95 (A-C), CREB1(D), and CDK5(E) in hippocampus of the long-term treatment with ginsengs extraction to rat brain. Data are expressed as the mean ± S.D., ** p <0.01 vs. the normal group, $$ p <0.01 vs. the SCOP group, $$$ p <0.01 vs. the SCOP group by ANOVA.
Discussion
Aging is the strongest risk factor for neurodegenerative diseases that cause cognitive decline and memory loss, such as Alzheimer's disease and dementia9). It has been reported that the incidence rate of dementia is about three times higher for those aged 63 and older than those younger than those younger than the age of 63, then doubled again at the age of 75, and increased to 1.5 times by the age of 847). As a result, the prevalence is expected to reach about 10% in the elderly over 65 years of age. Therefore, now when the aging population is accelerating, it is expected that problems such as medical problems, increase in social costs, and deterioration of quality of life related to cognitive decline and memory impairment occurring in the elderly population will continue to increase. It is recognized that the decrease in memory significantly reduces the quality of life, which is known to decrease rapidly in advanced diseases as well as in the early stages of diseases such as mild cognitive impairment.
In animal experiments, it was reported that administration of ginseng extract improved all steps in the effect of improving memory8), and it was reported that administration of ginseng was effective in recovering memory impairment due to its effect of enhancing learning and memory9). In another study, it was reported that the efficacy of ginseng not only improves memory ability but also improves subjective quality of life and mood14). Ginseng saponin has been suggested as a major active ingredient showing the efficacy of ginseng. It is called ginsenoside to distinguish it from other plant saponins. So far, about 30 types of ginsenoside have been isolated and their chemical structures have been elucidated. However, as for the various ginsenosides, different types of ginsenoside are activated depending on the treatment method in the process of processing ginseng into red ginseng or white ginseng. Studies so far have been mainly limited to red ginseng, which is a steamed and dried form of ginseng, and white ginseng, which is dried as it is. The purpose of this study is to compare and analyze the effects of red ginseng, white ginseng, and black ginseng on cognitive function and memory improvement.
In this study, to examine the cognitive function improvement effect after long-term administration of red ginseng extract, black ginseng extract, and white ginseng extract in experimental animals with long-term memory impairment caused by scopolamine administration, cognitive behavioral experiments were conducted using spatial perception learning tests such as water maze test, situational learning and an open space search activity test was performed to measure the anxiety state. Also, in the hippocampus, which is known to play a central role in spatial memory, the expression of PSD95 related to neural network reinforcement and the expression of CREB1 and Cdk5 related to memory increase were confirmed.
The scopolamine used in the experiment is a muscarinic cholinergic receptor antagonist and is widely used in experimental animal models used in research on memory and cognitive decline. It has been reported that scopolamine administration blocked cholinergic neurotransmission and decreased cognitive ability along with cholinergic dysfunction10). This has similar effects to the reduction of choline reuptake and acetylcholine synthesis in the hippocampus and cerebral cortex of Alzheimer's patients.
In the Morris water maze test, in the experimental group (SCOP) administered only with scopolamine, an increase in escape latency time taken to find the escape platform was observed during the 7-day training session, but red ginseng (SCOP+R) and black ginseng (SCOP+B) were administered. The average escape time of the receiving experimental group continued to decrease, and on the last 7 days, the result value was comparable to that of the control group (normal) (Fig. 2). Reduction of escape time is considered to indicate spatial perception learning ability related to long-term memory, and these results suggest that long-term administration of red ginseng and black ginseng is effective in improving long-term memory. It was clearly proven once again in the probe test, which was determined as a one-time test after the training period. It indicates whether the group remembers, and the crossing frequency in SCOP+R, SCOP+B, and SCOP+W groups all increased significantly compared to the SCOP group (Fig. 3A-3C). In the dwelling time in goal quadrant result, only the SCOP+B group increased significantly with the SCOP group (Fig. 3D).
In the exploratory behavior test when exposed to open space, there was no significant difference between the SCOP group administered with scopolamine alone, SCOP+R, SCOP+B, SCOP+W, and the control group administered with red ginseng, black ginseng, and white ginseng together. This suggests that long-term ginseng administration did not affect situational learning and anxiety. That is, it was possible to confirm the stability when the drug was administered for a long time. However, since situational learning and anxiety are not necessarily associated with a decrease or increase in search activity, additional research is needed on stability.
PSD95 is a structural protein located very close to the post-synaptic cell membrane and plays an important role in synapse formation and function, plasticity regulation, and neural network reinforcement11). Like BDNF, several studies have reported associations between the expression of PSD95 and neurological diseases. It has been reported that long-term memory impairment occurs when PSD95 expression is suppressed in the hippocampus of the developing brain12). It was also reported that PSD95 was associated with Alzheimer's and amnesia by observation in the hippocampus of subjects with cognitive impairment that PSD95 protein levels were decreased13). It has been reported that overexpression of PSD95 in hippocampal neurons not only induces maturation of glutamatergic synapses, but also induces postsynaptic clustering, activation of glutamate receptors and maturation of presynaptic terminals, thus playing a role in synaptic stabilization and neuroplasticity14,15). That is, the increase in PSD95 is related to the improvement of memory, and in this study, an increase in PSD95 expression was also observed in the group administered with red ginseng and black ginseng.
Cdk5 is a proline-directed serine/threonine kinase that is activated when interacting with the neuron-specific cofactors p35 or p3916). Cdk5 is implicated in numerous CNS processes, including cortical layer formation, neurotransmission, and mnemonic function17). Transgenic mice overexpressing the Cdk5 activator p25 also exhibit enhanced plasticity and memory formation18). Therefore, Cdk5 is a very important factor in increasing memory. Genetic variation in the CREB1 gene is of interest because the CREB signaling pathway contributes to the adaptation of neuronal properties in brain regions important for memory and executive function by regulating gene expression19). Age-related changes in the CREB signaling pathway have been reported in animal models, particularly in association with deficits in long-term and working memory, brain regions such as the hippocampus and limbic prefrontal cortex20). It has been consistently shown to increase CREB-dependent transcription and rescue deficient neuroplasticity and memory after inhibiting reactivation of the CREB signaling pathway in the hippocampus in young and old adult mice21). Similarly, the age-related decline in long-term memory found in control mice could be prevented by CREB gene transfer in the hippocampus22). Recent evidence also supports the level of CREB activation as a potential biomarker for cognitive decline in Alzheimer's disease23). In this study, long-term administration of red and black ginseng extracts to rats increased spatial perception learning and long-term memory. In particular, black ginseng showed an increase in Cdk5 protein expression (Fig. 6E) and red ginseng showed an increase in CREB1 in the hippocampus (Fig. 6D).
Summarizing the experimental results of this study, it was confirmed that long-term administration of ginseng was effective in improving cognitive function and memory. Food and health supplements including red ginseng and black ginseng can be effective against aging-related cognitive function and memory decline, demonstrating the possibility of developing new drugs with minimal side effects using them. Studies related to this are also being actively conducted, and Kyeongokgo containing ginseng ingredient can improve the memory loss state caused by cholinergic dysfunction, and the possibility of developing new natural materials. However, the etiology of diseases that show cognitive decline and memory loss, such as Alzheimer's, is not a single mechanism, but rather a complex combination of various factors such as amyloid accumulation, protein hyperphosphorylation, neurotransmitter abnormalities, oxidative damage, inflammatory response, and neurotrophic factor deficiency.
Conclusion
Ginseng extracts significantly restored the memory damaged by scopolamine suggesting that red ginseng increased the expression of CREB1 and PSD95 proteins, and black ginseng increased the protein expression of Cdk5 and PSD95 to induce memory recovery.
Acknowledgments
This study was supported by a grant from Wonkwang University 2023.
References
-
Tucker-Drob EM. Cognitive Aging and Dementia: A Life Span Perspective. Annual review of developmental psychology. 2019 Dec;1:177-96. PubMed PMID: 34046638. Pubmed Central PMCID: PMC8153102. Epub 2019/12/01. eng.
[https://doi.org/10.1146/annurev-devpsych-121318-085204]

-
Szakály Z, Szente V, Kövér G, Polereczki Z, Szigeti O. The influence of lifestyle on health behavior and preference for functional foods. Appetite. 2012 Feb;58(1):406-13. PubMed PMID: 22119479. Epub 2011/11/29. eng.
[https://doi.org/10.1016/j.appet.2011.11.003]

-
Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta medica. 1996 Feb;62(1):86-7. PubMed PMID: 8720394. Epub 1996/02/01. eng.
[https://doi.org/10.1055/s-2006-957816]

-
Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytotherapy research : PTR. 2008 Jul;22(7):851-8. PubMed PMID: 18567057. Epub 2008/06/21. eng.
[https://doi.org/10.1002/ptr.2384]

-
Wang LC, Wang B, Ng SY, Lee TF. Effects of ginseng saponins on beta-amyloid-induced amnesia in rats. Journal of ethnopharmacology. 2006 Jan 3;103(1):103-8. PubMed PMID: 16153793. Epub 2005/09/13. eng.
[https://doi.org/10.1016/j.jep.2005.07.010]

-
Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. Journal of ethnopharmacology. 1999 Aug;66(2):123-9. PubMed PMID: 10433468. Epub 1999/08/05. eng.
[https://doi.org/10.1016/S0378-8741(98)00190-1]

-
Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Archives of general psychiatry. 1998 Sep;55(9):809-15. PubMed PMID: 9736007. Epub 1998/09/15. eng.
[https://doi.org/10.1001/archpsyc.55.9.809]

- Zhang JT, Qu ZW, Liu Y, Deng HL. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chinese medical journal. 1990 Nov;103(11):932-8. PubMed PMID: 2177392. Epub 1990/11/01. eng.
-
Yamaguchi Y, Haruta K, Kobayashi H. Effects of ginsenosides on impaired performance induced in the rat by scopolamine in a radial-arm maze. Psychoneuroendocrinology. 1995;20(6):645-53. PubMed PMID: 8584605. Epub 1995/01/01. eng.
[https://doi.org/10.1016/0306-4530(95)00008-C]

-
Oh JH, Choi BJ, Chang MS, Park SK. Nelumbo nucifera semen extract improves memory in rats with scopolamine-induced amnesia through the induction of choline acetyltransferase expression. Neuroscience letters. 2009 Sep 11;461(1):41-4. PubMed PMID: 19463889. Epub 2009/05/26. eng.
[https://doi.org/10.1016/j.neulet.2009.05.045]

-
Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003 Dec 3;23(35):11270-8. PubMed PMID: 14657186. Pubmed Central PMCID: PMC6741048. Epub 2003/12/06. eng.
[https://doi.org/10.1523/JNEUROSCI.23-35-11270.2003]

- Wang SQ, Fang F, Xue ZG, Cang J, Zhang XG. Neonatal sevoflurane anesthesia induces long-term memory impairment and decreases hippocampal PSD-95 expression without neuronal loss. European review for medical and pharmacological sciences. 2013 Apr;17(7):941-50. PubMed PMID: 23640442. Epub 2013/05/04. eng.
-
Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer's disease. Journal of neuroscience research. 2010 Feb 15;88(3):469-77. PubMed PMID: 19774677. Pubmed Central PMCID: PMC2843415. Epub 2009/09/24. eng.
[https://doi.org/10.1002/jnr.22227]

-
El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science (New York, NY). 2000 Nov 17;290(5495):1364-8. PubMed PMID: 11082065. Epub 2000/11/18. eng.
[https://doi.org/10.1126/science.290.5495.1364]

-
Taft CE, Turrigiano GG. PSD-95 promotes the stabilization of young synaptic contacts. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014 Jan 5;369(1633):20130134. PubMed PMID: 24298137. Pubmed Central PMCID: PMC3843867. Epub 2013/12/04. eng.
[https://doi.org/10.1098/rstb.2013.0134]

-
Dhavan R, Tsai LH. A decade of CDK5. Nature reviews Molecular cell biology. 2001 Oct;2(10):749-59. PubMed PMID: 11584302. Epub 2001/10/05. eng.
[https://doi.org/10.1038/35096019]

-
Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. Journal of neurochemistry. 2006 Oct;99(2):353-70. PubMed PMID: 17029592. Epub 2006/10/13. eng.
[https://doi.org/10.1111/j.1471-4159.2006.04040.x]

-
Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. The European journal of neuroscience. 2003 Jul;18(2):423-31. PubMed PMID: 12887424. Epub 2003/07/31. eng.
[https://doi.org/10.1046/j.1460-9568.2003.02746.x]

-
Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends in neurosciences. 2010 May;33(5):230-40. PubMed PMID: 20223527. Epub 2010/03/13. eng.
[https://doi.org/10.1016/j.tins.2010.02.001]

-
Brightwell JJ, Gallagher M, Colombo PJ. Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiology of learning and memory. 2004 Jan;81(1):19-26. PubMed PMID: 14670355. Epub 2003/12/13. eng.
[https://doi.org/10.1016/j.nlm.2003.08.001]

-
Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002 Aug 29;418(6901):970-5. PubMed PMID: 12198546. Epub 2002/08/29. eng.
[https://doi.org/10.1038/nature00928]

-
Mouravlev A, Dunning J, Young D, During MJ. Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proceedings of the National Academy of Sciences of the United States of America. 2006 Mar 21;103(12):4705-10. PubMed PMID: 16537429. Pubmed Central PMCID: PMC1401230. Epub 2006/03/16. eng.
[https://doi.org/10.1073/pnas.0506137103]

-
Bartolotti N, Bennett DA, Lazarov O. Reduced pCREB in Alzheimer's disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Molecular psychiatry. 2016 Sep;21(9):1158-66. PubMed PMID: 27480489. Pubmed Central PMCID: PMC4995548. Epub 2016/08/03. eng.
[https://doi.org/10.1038/mp.2016.111]